Lab Tips
- only have ONE independent variable
- only have ONE dependent variable
- Discussions should talk about trends (increase/decreases)
- Discussions should talk about %error
- all graphs and tables should have titles
- a good title is descriptive, NOT ___ vs. __
Thermal Energy
Today we started a new section on Thermal Energy! |
| Which one do you think represents the higher temperature? |
Definitions
Thermal Energy - total kinetic and potential energy of all particles in an object.
Temperature - Average kinetic energy of particles in an object.
Absolute zero - the lowest possible temperature –273.15 °C or 0 K.
Kelvin - Unit of temperature. Same magnitude as °C, but starts at absolute zero.
Heat - Energy transfer from a hotter object to a cooler object, ∆E
Thermal Equilibrium - When two objects are in contact and the net exchange in energy is zero. (they are the same temp.)
 |
| Lord Kelvin! The unit of Kelvin was named after William Thomson, 1st Baron Kelvin. |
Question: What is the temperature in interstellar space?
Answer: 2.7 K
Answer: 2.7 K
 |
| Stephen Hawking is famous for many things, but one of them is showing that black holes have temperature! |
Methods of Thermal Energy Transfer
Conduction: direct contact
- touching a stove, chocolate melts in your mouth
Convection: particles in a fluid carry energy
- wind, boiling pot of water
Radiation: electromagnetic radiation to transfer energy
- sunshine feels warm, spot lights
Heat Capacity
The amount of energy it takes to change the temperature of an object by 1 K.
- different for every substance
- depends on mass
- measured in units of J / K
Specific Heat Capacity
The heat capacity for 1 kg of a substance.
- different for every substance
- measured in units of J / (K kg)
To find heat capacity, we use this equation
c = Q / m∆T
c = specific heat capacityHere are the specific heat capacities for common substances:
Q = heat (energy transferred, ∆E)
m = mass
∆T = temperature change, K
Using these values of c for many different substances, we can solve for Q or T.
When one substance loses energy, another one must gain it. This can be expressed as the following equation:
Q1 = - Q2
Or we can substitute in from the above and get:
Notice the final temperatures are the same on both sides. The system as reached thermal equilibrium.
 |
| The coffee and the cup might start at different temperatures, but eventually will reach the same final temperature. |
Homework
You should be able to get started on the homework from the Unit Outline, look for the Temperature and Heat section.

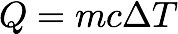
No comments:
Post a Comment