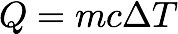Due to popular demand, here are a couple of quizzes for you to practice:
Friday, 28 November 2014
Thursday, 27 November 2014
Nov. 27 – Guest Speaker and Thinking Question Solutions
Today we have a guest speaker!
Here are the solutions to the thinking questions I gave out on Tuesday:
Here are the solutions to the thinking questions I gave out on Tuesday:
Quick Answers:
- The block does not make it to the top! You will get the final velocity is the square root of -9.6 therefore, there is no solution. If you calculate the highest it will go, you will get something close to 2 m up the last ramp.
- The height is 3.3 m.
Continue with your studying and keep asking me questions!
Tuesday, 25 November 2014
Nov. 25 – Review
Today: Review
Tomorrow: Wellness
Thursday: Guest Speaker
Friday: Review
Monday: Test
Test Topics
- Work, W=F∆d cos ø
- Work-Energy Theorem, W=∆E
- Kinetic Energy, E = 1/2 mv^2
- Gravitational Potential Energy, E = mgh
- Conservation of Energy, Ef = Ei
- Conservation of Energy with friction,
Ei = Ef + W (final energy is less)
- Power, P = ∆E/∆t
- Efficiency, eff = E(out)/E(in) x 100
- Heat transfer
- Thermal Energy, Q = mc∆T
- Thermal Energy Transfer, Q1 = -Q2
- Latent Heats, Lv = Qv/m , Lf = Qf/m
- Alpha, Beta, Gamma decays
- Fission and Fusion
- Half-life calculations
- Nuclear Reactors, Energy sources
- Matter Energy Equivalence, E=mc^2
Handouts
Textbook Review
Pg. 287-290 #3, 6, 8, 11, 13, 18, 20, 23, 24, 31, 32, 37, 38-40, 43-45, 47, 50-54, 58-60, 62
Don't try to do them all at once! You have a week until the test, space out your studying.
Monday, 24 November 2014
Nov. 24 – Fusion
Here is the schedule for the rest of the week:
Remember that this is for the producing of ONE He nucleus.
Ex) How much energy is produced from fusing 4g of helium (about 1 mole)?
4 grams of He is about 1 mole, which is 6.02 e 23 He nuclei. So the total energy is this number times the result from above.
- Monday - last lesson, extra help rm 245
- Tuesday - Review
- Wednesday - Wellness
- Thursday - Guest speaker, extra help rm 146
- Friday - Review
- Monday, Dec. 1 Test
First we wrapped up our discussion of fission by discussing the pros and cons of nuclear power.
Advantages and disadvantages of nuclear power
Pros:
- does not produce CO2, clean
- produces large amount of energy for small amount of fuel (high energy density)
- large reserves of nuclear fuel
Cons:
- warms up nearby lakes
- spent fuel is still radioactive
- dangerous to mine
- waste material from mining
Then we discussed fusion
Fusion
Lighter elements fuse together to form heavier elements
The sun fuses 4 H —> He in what is called the proton-proton chain.
The best fusion reactor we have is the sun.
Hydrogen mass: 1.008 amu = 1.6738 e -27 kg
Helium mass: 4.002 amu = 6.6455 e -27 kg
The mass of 4 H does not add up to 1 He. This was called the “mass defect”.
The missing mass is converted into energy.
E = energy
m = mass
c = speed of light, 3.00 e 8 m/s
Ex) How much energy is produced from the “mass defect”?
The mass lost is given by:
m = 4m(Hydrogen) – m(He)
m = 4(1.6738 e -27 kg) – (6.6455 e -27 kg) = 4.97 e -29 kgThis missing mass is converted into energy
E = mc²
E = (4.97 e -29 kg)(3.00 e 8 m/s)² = 4.47 e -12 J
Remember that this is for the producing of ONE He nucleus.
Ex) How much energy is produced from fusing 4g of helium (about 1 mole)?
4 grams of He is about 1 mole, which is 6.02 e 23 He nuclei. So the total energy is this number times the result from above.
E(total) = E x 1 mole
E(total) = (4.47 e -12 J)(6.02 e 23) = 2.69 e 12 J
This is a massive amount of energy!
A small amount of mass can be converted into a large amount of energy.
If we can do this in a fusion reactor we can produce useful energy with the only fuel being hydrogen!
Watch this video:
Homework
Complete these handouts
Friday, 21 November 2014
Nov. 21 – Halflife and Fission
Have a look at this simulation:
What happens? One particle can decay at any moment, but when you have hundreds or thousands (in real life, billions and billions) or particles we can find a characteristic time when half of the particles have decayed. This time is called the half-life. Once the time of the half-life has elapse, half of the sample remains. After another half-life, the sample is once again halved. A graph of the amount remaining looks like this:
- U-238 has a half life of 4.5 billion years.
- U-239 has a half life of 24 minutes.
- C-14 has a half life of 5700 years. (Used for carbon dating)
Here's the formula for calculating amount of material remaining:
Here are some examples:
Next we talked about fission:
Fission
A large nucleus breaks apart into smaller nuclei. This can be spontaneous (happens on its own) or induced (something “kicks off” the reaction).
 |
| Spontaneous. |
 |
| Induced. |
Induced fission can cause a chain reaction.
Nuclear Reactors
- Use enriched uranium to carry out a chain reaction producing thermal energy.
- Natural uranium has 0.7 % U-235
the rest is U-238
- Enriched uranium has 3-4% U-235
- Weapons grade: 90% U-235
 |
| How a nuclear power plant works. |
 |
| A simplified version of the image above. |
Moderator
- A substance used to slow down neutrons to control the chain reaction. graphite or heavy water.
Control Rods
- Cadmium or Boron is used to absorb neutrons to control the reaction.
- Nuclear bomb uses uncontrolled reactions to release massive amounts of energy.
Thursday, 20 November 2014
Nov. 20 – Radiation and Radioactive Decay
Today we discussed radiation. Here are the notes:
 |
| Marie Curie was one of the first people to study radiation. |
Radiation
Review:
proton - positively charged particle
neutron - neutral particle, same mass as proton.
electrons - negatively charged, 1000 times lighter
Isotopes
- isotopes are two or more atoms that have the same number of protons, but different number of neutrons.
 |
| Isotopes of lithium. |
Notation:
Use this notation for each element, mass number and proton number.
Example: This is how you would write krypton with a mass of 78.
How many protons and neutrons does it have?
Answer: Protons = 36, Neutrons = 78 – 36 = 42
What is radiation?
- radiation is high energy particles or high energy electromagnetic waves
 |
| Small amounts of radiation can be used to diagnose diseases such as in a PET scan. |
 |
| Here's what a chunk of radioactive uranium looks like. |
Nuclear Radiation
- radiation emitted from an unstable nucleus.
- the original nucleus is the “parent”
- the products are called “daughters”
- there are three types: alpha, beta, and gamma
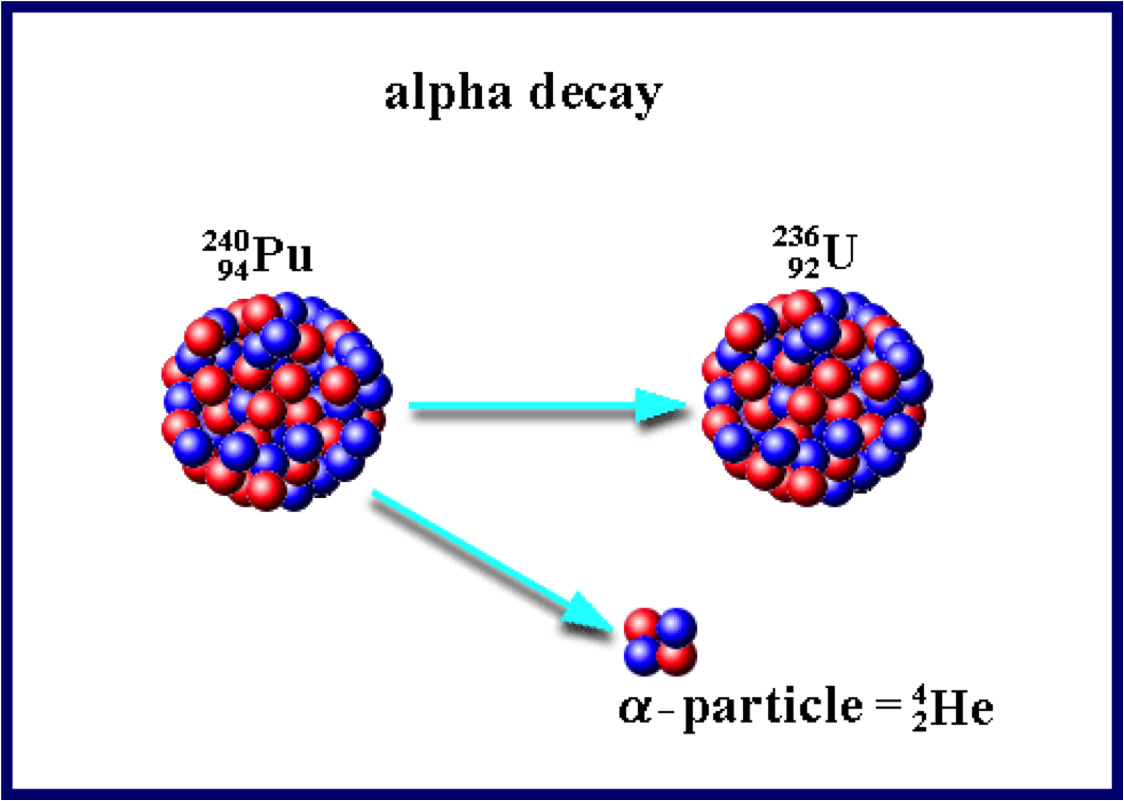
Alpha Decay
- an alpha particle consists of 2 protons and 2 neutrons (same as He nucleus)
- relatively large, easily stopped
- can penetrate a few cells into the body
Ex) Write the equation for Cu-64 undergoing alpha-decay.
Beta Decay
- A neutron decays into a proton, electron and anti-neutrino
- A beta particle is a high energy electron
- lighter and faster than alpha particles
- can be stopped by thin metal or plastic
Ex) Write the equation for Oxygen-19 undergoing beta-decay.
Gamma Decay
- A nucleus in an excited state loses energy and emits a photon
- A gamma particle is a high energy photon
Summary
Homework
- Please catch up on any homework you have not completed yet.
Wednesday, 19 November 2014
Nov. 19 – Change of State
At the beginning of class, I put a thermometer in a beaker of ice water. We measured the temperature every few minutes and I created a graph... here's what we got.
The beginning of the graph looks almost flat, but the last part started to spike up. What happened between 25 minutes and 30 minutes? Well, it turns out, through careful observation, that at this point, all the ice melted! The ice/water mixture was close to 0°C until the ice was completely melted.
It turns out that the energy added to the mixture was used to melt the ice rather than raise the temperature. In fact, if we extend this graph in both directions, we would get something of this shape:
We call the energy that goes into melting the ice the latent heat of fusion. This energy does not raise the temperature of the ice/water mixture, that's why the graph is flat (zero slope) at that point. The other flat area near the top is when the water boils and we call that the latent heat of vapourization.
Here are the notes:
Here are the terms you need to know:
Here are the different states of matter:
What is the temperature of this water?
Zero degrees! We know this because when ice and water are both present, the temperature must be zero degrees! If it was higher, it'd be all water, if it were colder, it'd be all ice.
Can water be solid, liquid and gas at the same time? Yes! It depends on the pressure. Here's the complicated graph I showed and tried to explain. You will NOT need to know this graph in this course. It's purely for the sake of interest.
You do need to know these example questions I did:
Continue with your textbook homework and remember your lab is due tomorrow!
The beginning of the graph looks almost flat, but the last part started to spike up. What happened between 25 minutes and 30 minutes? Well, it turns out, through careful observation, that at this point, all the ice melted! The ice/water mixture was close to 0°C until the ice was completely melted.
It turns out that the energy added to the mixture was used to melt the ice rather than raise the temperature. In fact, if we extend this graph in both directions, we would get something of this shape:
We call the energy that goes into melting the ice the latent heat of fusion. This energy does not raise the temperature of the ice/water mixture, that's why the graph is flat (zero slope) at that point. The other flat area near the top is when the water boils and we call that the latent heat of vapourization.
Here are the notes:
Latent Heat
The energy it takes to melt or freeze a substance is called the latent heat of fusion, QF
The energy it takes to evaporate or condense a substance is called the latent heat of vaporization, Qv
Specific Latent Heat of Fusion: LF = QF/m
Specific Latent Heat of Vaporization: Lv = Qv/m
- different for every substance
- measured in J/kg
Here are the terms you need to know:
Here are the different states of matter:
What is the temperature of this water?
Zero degrees! We know this because when ice and water are both present, the temperature must be zero degrees! If it was higher, it'd be all water, if it were colder, it'd be all ice.
Can water be solid, liquid and gas at the same time? Yes! It depends on the pressure. Here's the complicated graph I showed and tried to explain. You will NOT need to know this graph in this course. It's purely for the sake of interest.
You do need to know these example questions I did:
Continue with your textbook homework and remember your lab is due tomorrow!
Monday, 17 November 2014
Nov. 17 – Thermal Energy
Today I handed back your labs. Here are some tips for the next lab:
Use these tips to improve your next lab report. The next lab is due on Thursday!
Lab Tips
- only have ONE independent variable
- only have ONE dependent variable
- Discussions should talk about trends (increase/decreases)
- Discussions should talk about %error
- all graphs and tables should have titles
- a good title is descriptive, NOT ___ vs. __
Thermal Energy
Today we started a new section on Thermal Energy! |
| Which one do you think represents the higher temperature? |
Definitions
Thermal Energy - total kinetic and potential energy of all particles in an object.
Temperature - Average kinetic energy of particles in an object.
Absolute zero - the lowest possible temperature –273.15 °C or 0 K.
Kelvin - Unit of temperature. Same magnitude as °C, but starts at absolute zero.
Heat - Energy transfer from a hotter object to a cooler object, ∆E
Thermal Equilibrium - When two objects are in contact and the net exchange in energy is zero. (they are the same temp.)
 |
| Lord Kelvin! The unit of Kelvin was named after William Thomson, 1st Baron Kelvin. |
Question: What is the temperature in interstellar space?
Answer: 2.7 K
Answer: 2.7 K
 |
| Stephen Hawking is famous for many things, but one of them is showing that black holes have temperature! |
Methods of Thermal Energy Transfer
Conduction: direct contact
- touching a stove, chocolate melts in your mouth
Convection: particles in a fluid carry energy
- wind, boiling pot of water
Radiation: electromagnetic radiation to transfer energy
- sunshine feels warm, spot lights
Heat Capacity
The amount of energy it takes to change the temperature of an object by 1 K.
- different for every substance
- depends on mass
- measured in units of J / K
Specific Heat Capacity
The heat capacity for 1 kg of a substance.
- different for every substance
- measured in units of J / (K kg)
To find heat capacity, we use this equation
c = Q / m∆T
c = specific heat capacityHere are the specific heat capacities for common substances:
Q = heat (energy transferred, ∆E)
m = mass
∆T = temperature change, K
Using these values of c for many different substances, we can solve for Q or T.
When one substance loses energy, another one must gain it. This can be expressed as the following equation:
Q1 = - Q2
Or we can substitute in from the above and get:
Notice the final temperatures are the same on both sides. The system as reached thermal equilibrium.
 |
| The coffee and the cup might start at different temperatures, but eventually will reach the same final temperature. |
Homework
You should be able to get started on the homework from the Unit Outline, look for the Temperature and Heat section.
Wednesday, 12 November 2014
Nov. 12 – More on Power
Today I assigned you groups for the lab tomorrow. Remember to bring in any materials that you desire to conduct the lab. Paper, tape, timers and meter sticks will be provided.
Next I did another example on power using the mantis shrimp and then we discussed efficiency.
Next I did another example on power using the mantis shrimp and then we discussed efficiency.
Homework
- Continue with the textbook homework on the unit outline.
Tuesday, 11 November 2014
Nov. 11 – Power
Here are the solutions to the previous test.
Here's the lab I handed out today:
Over the next couple of days, find some material to construct your own ramp for this lab. It doesn't have to be fancy, some made out of paper or cardboard will work just fine.
Here are some answers to the Conservation of Mechanical Energy With Friction Worksheet.
The next topic is...
Power
 |
| The unit of power was named after this guy, James Watt. |
Here are some notes:
 |
| How much power does Usain Bolt generate? |
.jpg) |
| How much energy does your fridge use? |
Next we will do an example about the mantis shrimp:
Read this: http://theoatmeal.com/comics/mantis_shrimp
Homework
- Follow along with the unit plan. Do the homework from the Power section.
Subscribe to:
Comments (Atom)

















%2Bcopy.jpg)
-1.jpg)



-1.jpg)